incomplete octet of electrons|incomplete octet examples : Tagatay The second exception to the Octet Rule is when there are too few valence . Perna canaliculus), on Pulmonary and Respiratory Muscle Function in Non-asthmatic Elite Runners
incomplete octet of electrons,The second exception to the Octet Rule is when there are too few valence electrons that results in an incomplete Octet. There are even more occasions where the octet rule does not give the most correct depiction of a molecule or ion.
The second exception to the Octet Rule is when there are too few valence .
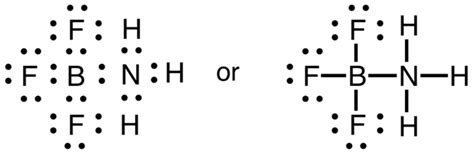
The problem with this structure is that boron has an incomplete octet; it only has six .The second exception to the Octet Rule is when there are too few valence electrons that results in an incomplete Octet. There are even more occasions where the octet rule .While molecules exist that contain atoms with fewer than eight valence electrons, these compounds are often reactive and can react to form species with eight valence .Incomplete Octet. There are certain atoms of certain elements that can exist in stable compounds forming bonds with less than eight valence electrons. When this occurs, the .
incomplete octet of electrons While most atoms obey the duet and octet rules, there are some exceptions. For example, elements such as boron or beryllium often form compounds in which the central atom is .
Too Many Electrons: Expanded Octets. Todd Helmenstine. Elements in periods greater than period 3 on the periodic table have a d orbital available with the same energy quantum number. Atoms in .incomplete octet of electrons incomplete octet examples The octet rule is a chemistry rule of thumb that says that atoms combine in a way that gives them eight electrons in their valence shells. This achieves a stable electron configuration similar to that of .
Exceptions to the Octet Rule. Many covalent molecules have central atoms that do not have eight electrons in their Lewis structures. These molecules fall into three categories: Odd .The Incomplete Octet. While most elements below atomic number 20 follow the octet rule, several exceptions exist, including compounds of boron and aluminum. Learning . The problem with this structure is that boron has an incomplete octet; it only has six electrons around it. Hydrogen atoms can naturally only have only 2 electrons in . While most atoms obey the duet and octet rules, there are some exceptions. For example, elements such as boron or beryllium often form compounds in which the central atom is .
Other articles where incomplete octet is discussed: chemical bonding: Incomplete-octet compounds: Less common than hypervalent compounds, but by no means rare, are species in which an atom does not achieve an octet of electrons. Such compounds are called incomplete-octet compounds. An example is the compound boron trifluoride, BF3, .

The Octet Rule. The other halogen molecules (F 2, Br 2, I 2, and At 2) form bonds like those in the chlorine molecule: one single bond between atoms and three lone pairs of electrons per atom.This allows each halogen atom to have a noble gas electron configuration, which corresponds to eight valence electrons. Incomplete Octet. In some compounds, the number of electrons surrounding the central atom in a stable molecule is fewer than eight. Beryllium is an alkaline earth metal and so may be expected to form ionic bonds. However, its very small size and somewhat higher ionization energy compared to other metals actually lead to . Exceptions to the octet rule fall into one of three categories: (1) an incomplete octet, (2) odd-electron molecules, and (3) an expanded octet. Incomplete Octet. In some compounds, the number of electrons surrounding the central atom in a stable molecule is fewer than eight. Beryllium is an alkaline earth metal and so may be .
Exceptions to the octet rule fall into one of three categories: (1) an incomplete octet , (2) odd-electron molecules , and (3) an expanded octet. Incomplete Octet. In some compounds, the number of electrons surrounding the central atom in a stable molecule is fewer than eight. Beryllium is an alkaline earth metal and so may be expected to form .The "octet rule" says that in many compounds the most stable (correct) electron configuration is when there are 8 electrons (four filled orbitals). This is a consequence of the fact that many compounds involve the s and p block electrons, which contribute 4 orbitals and can thus contain 8 electrons. We saw how we can predict the number of .Incomplete Octet. There are certain atoms of certain elements that can exist in stable compounds forming bonds with less than eight valence electrons. When this occurs, the atom of the element within the molecule is said to contain an incomplete octet. The common examples of such elements are hydrogen (stable with only 2 valence electrons .

Exception 2: Incomplete Octets. The second exception to the Octet Rule is when there are too few valence electrons that results in an incomplete Octet. There are even more occasions where the octet rule does not give the most correct depiction of a molecule or ion. This is also the case with incomplete octets.
The octet rule is a bonding theory used to predict the molecular structure of covalently bonded molecules. According to the rule, atoms seek to have eight electrons in their outer—or valence—electron shells. Each atom will share, gain, or lose electrons to fill these outer electron shells with exactly eight electrons. Exception 2: Incomplete Octets. The second exception to the Octet Rule is when there are too few valence electrons that results in an incomplete Octet. There are even more occasions where the octet rule does not give the most correct depiction of a molecule or ion. This is also the case with incomplete octets.Exception 2: Incomplete Octets. The second exception to the Octet Rule is when there are too few valence electrons that results in an incomplete Octet. There are even more occasions where the octet rule does not give the most correct depiction of a molecule or ion. This is also the case with incomplete octets.incomplete octet examplesExceptions to the octet rule fall into one of three categories: (1) an incomplete octet, (2) odd-electron molecules, and (3) an expanded octet. Incomplete Octet. In some compounds, the number of electrons surrounding the central atom in a stable molecule is fewer than eight. Beryllium is an alkaline earth metal and so may be expected to form .
The second exception to the Octet Rule is when there are too few valence electrons that results in an incomplete Octet. There are even more occasions where the octet rule does not give the most correct depiction of a molecule or ion. This is also the case with incomplete octets. Species with incomplete octets are pretty rare and . Exception 2: Incomplete Octets - Carbocations. The second exception to the octet rule is when there are too few valence electrons on one atom, which results in an incomplete octet. Species with incomplete octets are pretty rare and generally are only found in some beryllium, aluminum, and boron compounds including the boron hydrides.Exception 2: Incomplete Octets. The second exception to the Octet Rule is when there are too few valence electrons that results in an incomplete Octet. There are even more occasions where the octet rule does not give the most correct depiction of a molecule or ion. This is also the case with incomplete octets.The bonding in carbon dioxide (CO 2): all atoms are surrounded by 8 electrons, fulfilling the octet rule.. The octet rule is a chemical rule of thumb that reflects the theory that main-group elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas.The rule is .
The second exception to the Octet Rule is when there are too few valence electrons that results in an incomplete Octet. There are even more occasions where the octet rule does not give the most correct depiction of a molecule or ion. . The most contributing structure is probably the incomplete octet structure (due to Figure 8.7.5 being .
incomplete octet of electrons|incomplete octet examples
PH0 · octet rule violation
PH1 · incomplete octet examples
PH2 · boron octet rule exception
PH3 · Iba pa